MixturesYesterday in class we reviewed the various ways of separating mixtures. A few of the most commonly used methods to separate homogeneous mixtures are distillation filtration and chromatography.
- Figure A is showing distillation, where the substance's state is changed from a liquid to a gas and back to a liquid by boiling the liquid substance.
- Figure B is showing filtration, which can be used to separate solid and liquid parts of a mixture
- Figure C is showing the results of chromatography, where a solution can be separated by allowing it to flow along a stationary substance
Atoms
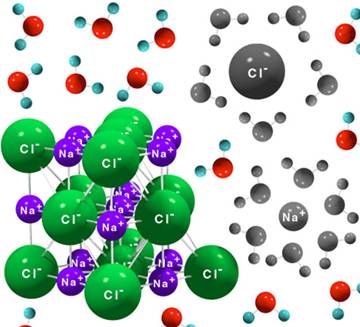
- matter is made up of atoms
- molecules are groups of atoms held together by electrical bonds
- ions are atoms or molecules that have an electric charge
- (+) cations(remember, cations are PUSSYtive)
- (-) anions
- atoms are made up of 3 sub-atomic particles
- protons have a positive charge, are inside the nucleus, each element has a different number of protons and the number of protons in an element is called its atomic number
- neutrons are neutral, they are inside the nucleus, have often the same mass as protons and adding or removing neutrons doesn't change the element
- electrons have a negative charge, are located outside of the nucleus, are 1800 times smaller than protons and chemical reactions occur between electrons in different atoms/compounds
Periodic Table
.. just a few quick facts/reminders about the PT
- Families(or groups) form vertical columns, all elements of a family have similar traits/characteristics
- Periods are horizontal rows. Elements gradually change from metals to non-metals as you move from left to right.
- most elements use Latin names as well(some exceptions are copper-cuprum, gold-aurum, iron-ferrum, lead-plumbum and silver-argentum)
Naming Nomenclatur
Naming chemical compounds has been a very difficult task and different systems have been used through the centuries. Today the most common system is IUPAC for most chemicals(ions, binary ions, polyatomic ions, molecular compounds and acids)
When you are naming ions remember there are metals, non-metals and polyatomic ions. For metals, use the name of the element and add the ion(AL+3 = aluminum ion). For non-metals, remove the original ending and add -ide(F=fluorine to fluoride). And remember that polyatomic ions have special names.
When you are creating binary ionic compounds, know that they contain a metal and a non-metal. Also know that metallic and non-metallic ions bond together, that the electron is transferred from the metal to the non-metal and the net charge must always be zero.
This is a handout you can use to practice naming and writing chemical formulas




.bmp)










 B)
B) C)
C)