we learned that matter:
- is anything that has mass and occupies space
- can exist in many different states, but the most common are
- solid, liquid and gas
- plasma (in stars, can be aqueous or amorphos)
solids: has definite shape and volume
liquids: can change shape but has definite volume
gases: can change shape and volume
aqueous: something dissolved in water
e.g. NaCl(s) ----> NaCl(aq)


We also learned that matter can undergo many changes, and nearly all of them can be classified as physical, chemical or nuclear
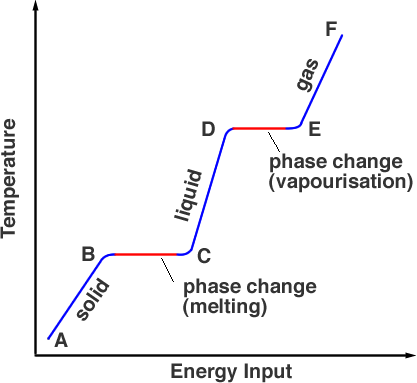
***a note about phase changes ***
- changing from solid to gas can often be confused as a chemical change
- the chemicals remain the same
in a physical change:
-no new substances are formed (like boiling water, cutting wood, and smashing cars)
- involves changing the shape or state of matter (crushing, tearing, etc)
in a chemical change:
- new substances are formed
- properties of matter change (conductivity, acidity, colour, etc)
eg. iron rusting, burning water, digesting
here is a video of physical changes and chemical changes:
conservation of matter:
- in physical and chemical changes matter is NEVER created or destroyed. ever.
- this is called conservation of matter
here's a diagram to help with the idea of conservation of matter:

Thats all for now!

No comments:
Post a Comment