Electrical conductivity in solutions requires charged ions to be transferred.
Iionic solutions dissociate or form ions so they always conduct.
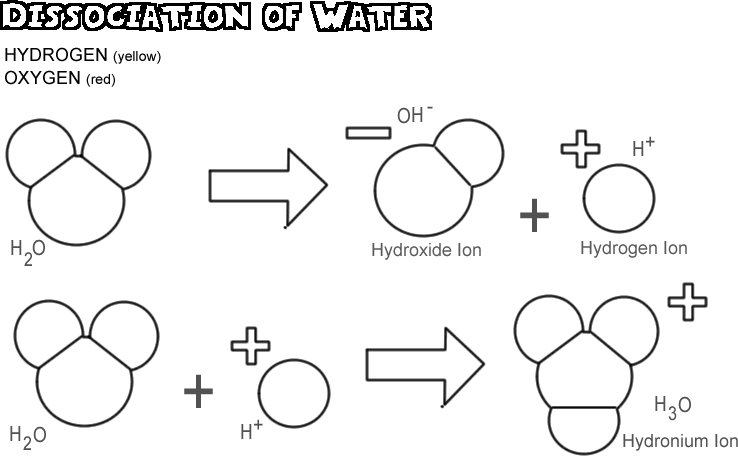
Molecular substances do not usually dissociate.
follow the chart to determine conductivity:
Iionic solutions dissociate or form ions so they always conduct.

Molecular substances do not usually dissociate.
follow the chart to determine conductivity:
 Examples
Exampleselement - yes/no - reason
Mercury - yes - metal
Carbon - no - solid non-metal
NaCl - yes - ionic
acetic acid - yes - acid
Iodine - no - not ionic/acid/base/metal

No comments:
Post a Comment