- reactions that release heat are exothermic
- reactions that absorb heat are endothermic
- heat IS a form of energy
- all chemicals have stored energy, also known as enthalpy
- enthalpy is given the symbol 'H'
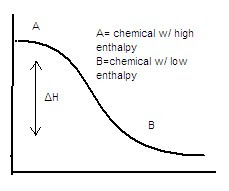
- ΔH is the change in enthalpy
Enthalpy Diagrams
Exothermic
- high to low enthalpy
- ΔH is negative
- heat is released
Endothermic
- low to high enthalpy
- ΔH is positive
- heat is absorbed
wanna learn more about enthalpy change? click here....... you know you waaaaaannaa
--
this is totally off topic but i think Mr. Doktor will find this pretty cool. well at least i think its pretty awesome..its a tv show on discovery or FSN called sport science. Hope you enjoy :)


No comments:
Post a Comment